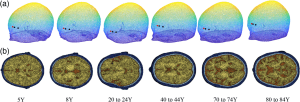
In our latest work in Neurophotonics, Hongting Zhao determines the influence of oversimplifying the head geometry on brain blood flow estimated with diffuse correlation spectroscopy (DCS). Due to the noninvasive nature of DCS measurements, light must pass through extracerebral layers (i.e., skull, scalp, and cerebral spinal fluid) before detection at the tissue surface. To minimize the contribution of these extracerebral layers to the measured signal, an analytical model has been developed that treats the head as a series of three parallel and infinitely extending slabs (mimicking scalp, skull, and brain). The three-layer model has been shown to provide a significant improvement in cerebral blood flow estimation over the typically used model that treats the head as a bulk homogenous medium. However, the three-layer model is still a gross oversimplification of the head geometry that ignores head curvature, the presence of cerebrospinal fluid (CSF), and heterogeneity in layer thickness. Using Monte Carlo modeling in a four-layer slab medium and a three-layer sphere medium to isolate the influence of CSF and curvature, respectively, we found both head curvature and failing to account for CSF lead to significant errors in the estimation of cerebral blood flow. However, the effect of curvature and CSF on relative changes in blood flow is minimal. In sum, these findings suggest that the three-layer model holds promise for improving estimation of relative changes in cerebral blood flow; however, estimations of absolute cerebral blood flow with the approach should be viewed with caution given that it is difficult to account for appreciable sources of error, such as curvature and CSF.